Only three individuals had received two Nobel Prizes in science until Barry Sharpless did it again this year, with his groundbreaking research in chemistry. Another Nobel laureate this year also joined the multigenerational Nobel Prize club.
Almost from the moment it was awarded for the first time in 1901, the Nobel Prize became the most prestigious and important award in the scientific world. Nobel laureates belong to an exclusive club of scientists. Over its 122-year history, 611 male and 23 female scientists have received prizes in science: 225 in physiology or medicine, 222 in physics, and 191 in chemistry. Within this club is an even smaller group: those who have been awarded a Nobel Prize in science twice. Until now, only three scientists belonged to this unique group; this year— this year—after a 42-year gap—another scientist has joined their ranks.
Two Prizes for the Same Research
The founder of this exclusive club was French physicist Marie Curie.Soon after Henri Becquerel discovered radioactivity in 1896, Curie and her husband Pierre began investigating this phenomenon to determine its source While trying to isolate the source of radiation from uranium ores they realized that the ores themselves contained additional radioactive elements, and managed to isolate two of them. In 1903, just the third year of the Nobel Prize’s existence, the committee decided to award the prize jointly to Becquerel and Pierre Curie. But Pierre Curie was a man of conscience and backbone and informed the committee that he would not accept the prize unless it was also awarded to his wife, who had led the research. The committee relented: half of the prize was awarded to Becquerel and the other half was shared between Pierre and Marie Curie, making her the first woman to receive a Nobel Prize. This was also the first time the prize was shared among three laureates.
Eight years later, in 1911, the committee decided to award Marie Curie the Nobel Prize in Chemistry for the discovery of the two radioactive elements, which she named radium, for its radioactive properties, and polonium, after her homeland, Poland. This time the prize was awarded to her alone, as Pierre had been killed in a car accident in 1906. This was the first, and so far the only, instance of a scientist being awarded Nobel Prizes in two different fields for discoveries made during the same research.
For 61 years, Marie Curie remained the only person to have been awarded two Nobel Prizes in science, until 1972, when the American physicist, John Bardeen, joined this exclusive club.
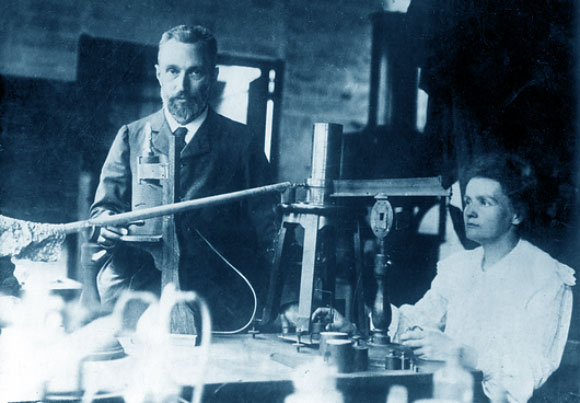
Three Nobel Prizes in one photo. Marie and Pierre Curie in their laboratory, 1905 | Photo: UNIVERSAL HISTORY ARCHIVE\UIG / SCIENCE PHOTO LIBRARY
Transistors and Superconductivity
John Bardeen was a brilliant physicist who left academia after World War II to work at Bell Telephone Laboratories, part of the AT&T telecommunications company. There, along with his manager, William Shockley, and colleague, Walter Brattain, he developed the first transistor in 1947, sparking a revolution in electronics and transforming all aspects of life. In 1956 the three shared the Nobel Prize in Physics for this invention, although their collaboration had already ended. Shockley, who believed the invention was primarily his, resented the credit given to his partners and increasingly distanced himself from them. Discontented with this environment, Bardeen returned to academia in 1951 and became a professor at the University of Illinois.
At Illinois, Bardeen’s research focused on superconductivity—a state in which a conductive material loses all resistance, allowing electrons to flow without energy loss or heat. Together with his students Leon Cooper and Robert Schrieffer, Bardeen uncovered the physical mechanism behind this phenomenon, discovering that in the state of superconductivity electrons form pairs that do not exchange energy with the metal, enabling resistance-free movement. This theory was named BCS, after the initials of its three formulators.
When Bardeen received the Nobel Prize in 1956, the King of Sweden jokingly scolded him for bringing only one of his three children to the ceremony. Bardeen responded with a promise to bring them all next time. Surprisingly, he fulfilled this promise when he, Cooper, and Schrieffer were awarded the Nobel Prize in Physics in 1972 for the BCS theory.
When Bardeen received the Nobel Prize in 1956, the King of Sweden jokingly scolded him for bringing only one of his three children to the ceremony. Bardeen responded with a promise to bring them all next time. Surprisingly, he was able to fulfill his promise when he, Cooper, and Schrieffer were awarded the Nobel Prize in Physics in 1972 for the BCS theory.Schrieffer with the Nobel Prize in Physics in 1972.

The second time he brought all his children. John Bardeen, the only one to receive the Nobel Prize in Physics twice | Photo: SCIENCE PHOTO LIBRARY
Proteins and DNA
Two years after Bardeen was awarded his first Nobel Prize, the Nobel Prize in Chemistry was awarded to Frederick Sanger, a British biochemist who developed an efficient method for determining the sequence of amino acids in proteins. All proteins are composed of the same building blocks—twenty amino acids. Despite their complex three-dimensional structures, each protein is made up of a single long chain of amino acids that folds into its active form after assembly. Sanger developed a substance that breaks down the protein sequence into short segments, then used a combination of chemical methods to determine the composition of these segments and decipher the entire protein sequence. This breakthrough made it possible to better understand a protein's three-dimensional structure and identify the gene responsible for its production. He demonstrated the method on insulin, which is used to treat diabetes, an achievement that earned him the Nobel Prize in Chemistry at just 40 years old.
In the following years, Sanger gradually transitioned from studying protein sequences to studying the sequences of nucleic acids, which dictate the structure and sequence of proteins. IHe first developed a method to determine the sequence of bases in RNA and later found a way to sequence the bases in a DNA molecule, using enzymes that construct the helices and radioactively labeled bases. Although complex, this method enabled Sanger and his colleagues, in 1977, to sequence the complete DNA of the virus ΦX174 - the first organism to be sequenced. This achievement earned him the Nobel Prize in Chemistry in 1980, or rather a quarter of the prize: he shared half of the prize with Walter Gilbert, who developed a different method for determining the sequence; while the remaining half was awarded to Paul Berg, who developed techniques for cutting and joining DNA, and thus laid down the foundations for genetic engineering.
The exclusive club of two-time Nobel Prize laureates sometimes also includes Linus Pauling. Pauling was awarded the Nobel Prize in Chemistry in 1954 for discoveries that helped explain how chemical bonds form between atoms and molecules. Using his status, Pauling later campaigned tirelessly against nuclear weapons testing, collecting signatures from 11,000 scientists worldwide for a petition he submitted to the United Nations Secretary-General. His efforts contributed significantly to the Partial Nuclear Test Ban Treaty between the USA, the Soviet Union, and Britain, which prohibited nuclear weapons testing, and earned him the Nobel Prize in Peace in 1962.
Unlike the other Nobel Prizes, which are awarded only to individuals, the Nobel Peace Prize can also be given to organizations. The International Red Cross has received it three times, and the United Nations High Commissioner for Refugees twice. While Pauling is a scientist who won two Nobel Prizes, since only one was in science, he is generally not included in the exclusive club of two-time Nobel Prize laureates in science. This club remained limited to three members until 1980. And then came Sharpless.

Frederick Sanger received his first Nobel Prize for determining the amino acid sequence of proteins and the second for determining the base sequence of DNA. Sanger in his laboratory in 1989 | Photo: CORBIN O’GRADY STUDIO / SCIENCE PHOTO LIBRARY
Rare Fish
Karl Barry Sharpless was born in Philadelphia on April 28, 1941. He grew up in the city, but spent his vacations at his family’s beach house in New Jersey, where he fell in love with fishing and sailing. “...unlike most fishermen, I cared less for the size or quantity of the catch than for its rarity”, he related later on.
In high school, Sharpless did not show special interest in science, and upon finishing it he enrolled in college with the intention of studying medicine, like his father. But during his studies at Dartmouth College, Prof. Tom Spencer noticed the brilliant student, recruited him for a project in his laboratory, and upon his graduation convinced him to try a PhD in chemistry instead of continuing to medical school. He even sent Sharpless to his own PhD supervisor, the organic chemist Eugene van Tamelen from Stanford University. After completing his PhD and two post-doctoral fellowships, Sharpless was accepted to the faculty of the Massachusetts Institute of Technology (MIT) in 1970, where his research focused on catalysts of chemical reactions.
As its name implies, a catalyst is a substance that increases the rate of chemical reactions, which would occur very slowly in its absence. While the catalyst participates in the reaction, it is not consumed during the reaction, nor does it change its state; thus a small amount of catalyst can function for a long period of time or with a large amount of products and reactants. Therefore, catalysts are of enormous importance for the chemical industry.

Was convinced to study chemistry instead of medicine. Barry Sharpless in 2018 | Photo: Bengt Oberger, Wikipedia, CC BY-SA 4.0
Asymmetric Chemistry
Sharpless’s research focused on catalysts for a certain type of chemical processes, known as chiral reactions. Such reactions produce molecules in two forms, which are structurally identical, but constitute exact opposites with respect to their spatial arrangement, like mirror images of each other. They can be compared to two hands - they are identical to each other but they are not congruent. We cannot place one of our hands on top of the other and obtain a complete overlap. Therefore, these molecular forms are termed “left” and “right”.
In spite of the fact that the two chiral molecules are practically identical in terms of their chemical structure, save for constituting a mirror image of each other, there are surprising differences in their biological activity. For example, the right-handed form of the aromatic molecule limonene, smells like citrus, while the left-handed form of the same molecule smells like pine, since each of them reacts with different receptors in our olfactory system.
Chirality can also have dangerous consequences. The most well-known example is that of thalidomide—a drug that was approved during the mid-20th century for the treatment of morning sickness during pregnancy. Many women who took the drug gave birth to babies with severe developmental defects, and the investigation found out that while the right-handed molecule does indeed prevent morning sickness, the left-handed molecule has a severe impact on fetal development. Today, we know about other similar medications, thus there is a need for chemical processes that produce only one of the two forms of a chiral molecule.
Sharpless’ research focused on catalysts of such asymmetric reactions, which would produce only one form of a chiral molecule. He focused on substances called alkenes - molecules with at least one double bond between two carbon atoms. He searched for catalysts for oxidation reactions that would enable to obtain only the desired form of certain alkenes, and in 1980 he developed a process for producing the right-handed form of molecules known as epoxides, through oxidation and use of titanium atoms as catalysts. This process, which today is named after him, is very important in many chemical industries. Among them, the pharmaceutical industry, since such epoxides are used as a starting material in the production of beta blockers, drugs used to treat a wide range of diseases of the heart, blood vessels and of the nervous system.
This development earned Sharpless half of the Nobel Prize in Chemistry in 2001. The second half was shared by William Knowles and Ryoji Noyori, who developed a different process for obtaining a specific form of a chiral molecule, based on the addition of hydrogen atoms to the molecule, rather than on its oxidation. A few months prior to winning the Nobel Prize in 2001, Sharpless (together with Noyori) was also awarded the Wolf Prize, the most prestigious international prize in science awarded in Israel, which is considered a good predictor for the Nobel Prize, since many of its laureates in relevant fields are later awarded a Nobel Prize.

Preferring a certain form. Sharpless (left), Noyori (center) and Knowles in a joint interview for the Nobel Prize website, December 2001 | Source: William S. Knowles - Interview. NobelPrize.org
Connecting With a Click
At the time he received the Nobel Prize, Sharpless was already deep into a slightly different research field. About a decade earlier he returned to the West Coast of the United States and began working as a researcher at The Scripps Research Institute in San Diego. He gradually shifted his field of research there to the search for ways to make chemical processes more efficient. In 1998 Sharpless coined the phrase “click chemistry” and in 2001 he defined it for the first time in a paper he authored with two of his colleagues.
The problem that he pointed out in the article was the great difficulty in producing certain materials. Chemists today can synthesize almost any molecule produced in nature, and even those that aren’t, but in many cases these processes are complicated, requiring a sequence of chemical reactions, each of which may require a different environment. In addition, these processes often yield many byproducts that may inhibit the reaction itself. Due to that, the resulting processes are long, expensive, and often also polluting. The situation gets even more complicated when a process that worked well in the laboratory needs to be upscaled to an industrial scale. For example, the authors brought the example of the antibiotic meropenem, which required no less than six years of development in order to be produced at a large scale.
Sharpless and his colleagues emphasized in their paper that one of the main problems in the production of materials lies in the creation of bonds between carbon atoms. They suggested bypassing the problem by replacing the problematic processes with other reactions that bind a carbon atom to another atom, such as nitrogen or oxygen, and to bind this atom to a second carbon atom. Thus a ‘bridge’ is formed, connecting the two carbon atoms without the need to bind them together directly. They claimed that although the molecules formed in this way are not identical to the original molecules that are formed in nature, it would still be possible to produce molecules that perform the same function, using substances that connect them easily, in an aqueous environment, in processes that can be conducted on a large scale in a simple, cheap and environmentally friendly way.
The notable disadvantage of the click chemistry method was in the relatively limited number of chemical reactions that met its many criteria. The authors themselves defined this as follows: “the click chemistry method is defined by, enabled by, and limited by a handful of nearly perfect chemical reactions”. This problem was solved, at least partially, already in 2002, with the discovery of a chemical reaction that meets these requirements perfectly, and is associated today with click chemistry more than any other reaction.
Triazoles are compounds that contain a pentagonal ring, consisting of three nitrogen atoms bound to each other and two carbon atoms that are bound to two of the nitrogen atoms, with a double bond between the carbon atoms. Triazoles constitute important core structural components or starting materials in the production of drugs, industrial chemicals as well as products used in agriculture, but their large-scale production was expensive and complicated. Sharpless and his colleagues found that triazoles can be produced easily and efficiently by a reaction between azide molecules, which contain three nitrogen atoms, and alkynes, which contain carbon atoms with a triple bond between them, with the help of copper atoms that function as catalysts. This reaction can take place in water, and paves the way for the production of a great range of compounds.

In parallel to the work done by Sharpless, a team of researchers from Denmark discovered the same exact reaction. Morten Meldal, born in 1954, studied chemical engineering at the Technical University of Denmark in Copenhagen, where he also completed his PhD in carbohydrate synthesis. Following post-doctoral research at Cambridge, he returned to the university as a researcher, and later moved to the University of Copenhagen. His research there focused on searching for new compounds for medications: he produced an enormous number of compounds, and tested their activity against disease causing agents.
In one of his experiments, Meldal used compounds that form by a connection between alkynes and molecules known as acyl halides. When this chemical process, which was supposed to be relatively simple, did not yield the expected compound, Meldal examined what had happened, and was surprised to discover that the alkyne reacted with the opposite side of the molecule, which contains three nitrogen atoms, and that this process occurs with surprising ease.
Carolyn Bertozzi was born in Boston in 1955. She studied chemistry at Harvard University, and at the same time she played in several bands, including Bored of Education, together with Tom Morello, who later became the guitarist of Rage Against the Machine. In 1993 she received her PhD from Berkeley University, where she was later appointed as a researcher. Her research focused on biochemistry, but in contrast to most biochemists, who study proteins, DNA or RNA, she chose to focus on glycans - sugar-based molecules that play important roles in many biological processes.

From a rock band to anti-cancer treatments. Carolyn Bertozzi | Photo: Christopher Micel, Cmichel67, Wikipedia, CC BY-SA 4.0
Bertozzi wanted to study how glycans bind to proteins, fats or other sugars, in order to understand processes such as immune responses, but encountered difficulty with finding chemical processes for these bonds that didn’t impact the function of the cell itself. Her research gave rise to a new field of research, bioorthogonal chemistry, which focuses on biochemical processes in living systems without interfering with the native biological processes.
Bertozzi realized that the reaction developed by Sharpless and Meldal could help her, and developed a method in which the click reaction could take place without copper or any other toxic metal, so that it could act in a living cell without affecting its activity. Now Bertozzi could label the glycans quite easily. She provided the cells with sugar molecules that carried an added azide group and the cells utilized this sugar for their needs, incorporating it also within the glycans found on the cell surface. Subsequently, it was possible to easily connect a wide range of molecules to these sugars, using alkynes found in external molecules. Thus, for example, she connected a fluorescent molecule to the glycans on the cell surface, and could then use a microscope to follow the bridging between the glycans and other compounds, without affecting cell function.
Bertozzi’s research led, among other things, to the development of cancer treatments. Many other researchers also used the reactions of click chemistry to develop many diverse molecules that are used in biological research and in drug development.
The discoveries of Sharpless, Meldal and Bertozzi, which paved the way for efficient, cheap, and relatively non-polluting chemical processes and to important progress in biological and medical research, earned them this year with the Nobel Prize in Chemistry. The prize will be divided in equal parts between the three. Bertozzi is the eighth woman in total to be awarded the Nobel Prize in Chemistry, but the fourth in the last four years. She, too, received the Nobel Prize after also winning the Wolf Prize this year.
As mentioned above, with his second Nobel Prize, Sharpless joins the exclusive club—which currently numbers only four people—who have received two Nobel Prizes in science. “Winning for a second time is absolutely thrilling, and it’s so gratifying that it’s happening in my lifetime”, he told reporters following the announcement of the prize. “I’ve always been lucky, but right now I think I’m the luckiest person”.

Marking sugars in the a cell without affecting the natural processes occurring within it. Bioorthogonal chemistry | Illustration: Johan Jarnestad/The Royal Swedish Academy of Sciences ©
Medicine: Genetics of Prehistoric Man
Sharpless, Bertozzi and Meldal received the Nobel Prize at a ceremony in Stockholm, which takes place each year on December 10, the anniversary of the death of the founder of the Nobel foundation, Alfred Nobel. This year, the recipients of the prizes in science included two Scandinavians; alongside Meldal, from Denmark, stood Svante Pääbo, from Sweden, who was awarded the Nobel Prize in Physiology or Medicine for genetic research of extinct species in the human lineage
In 1986 Pääbo obtained his PhD from Uppsala University in Sweden, for a research study on viruses. But already during his studies he became interested in Ancient Egypt, and managed to extract DNA from a 2,400-year-old mummy. Later in his career, at the University of California, Berkeley, and at the University of Munich, he developed sophisticated methods for extracting even more ancient DNA; he managed to study the genome of Neanderthal Man and also to show that modern humans carry Neanderthal genes, indicating intriguing encounters that occurred between our ancient forefathers and ancient foremothers with Neanderthals, tens of thousands of years ago.
In other studies, Pääbo and his colleagues discovered an unknown species of prehistoric man owing to the analysis of the DNA extracted from bones found in the Denisovan cave in Siberia. It was thus given the name, Denisovan Man. Pääbo even managed to extract DNA of the Neanderthal Man and the Denisovan Man from soil samples taken from the cave, without the need for any direct remnants such as bones or teeth.
Pääbo is only the third laureate of the Nobel Prize in Physiology or Medicine in the last two decades to receive the award alone, without partners. His win also expands a limited club: his father, Sune Bergström, received the Nobel Prize in Physiology or Medicine in 1982 for his study of prostaglandins, and Pääbo is only the eighth person to win a Nobel Prize after one of his parents. This club includes some very famous names, such as Irène Joliot-Curie, who was awarded the Nobel Prize in Chemistry in 1935, after her two parents, Marie and Pierre Curie; the physicist Niels Bohr, who was awarded the Nobel Prize in Physics in 1922, and his son Ove, laureate of the Nobel Prize in Physics in 1975; J. J. Thomson, who was awarded the Nobel Prize in Physics in 1906 and his son George who was laureate of the Nobel Prize in Physics in 1937; and Arthur Kornberg, who was awarded the Nobel Prize in Physiology or Medicine in 1959, and his son Roger, laureate of the Nobel Prize in Chemistry in 2006.

A Nobel Prize after his father, for research on prehistoric man. Svante Pääbo | Photo: Duncan.Hull, Wikipedia, CC BY-SA 4.0
Physics: Demonstrating Quantum Principles
The Nobel Prize in Physics was shared this year between three researchers who showed that quantum entanglement is indeed possible. Quantum entanglement is a phenomenon in which particles are found in a shared quantum state, and when an action is conducted on one of them, it is also conducted on the other, regardless of the distance between them. This seems to be a violation of a basic law of physics, since if we consider the two particles to be separate entities, an immediate action on a very distant entangled particle transmits information between them at a speed greater than the speed of light.
In the mid-20th century, the British physicist, John Stewart Bell, developed a series of inequalities that demonstrated an upper limit to the amount of information that can be extracted from two sources of information. According to the predictions of Quantum Theory, quantum entanglement can be used to obtain more information than the maximum possible amount of information according to the Bell inequality. In other words, quantum entanglement allows performance of an act that is mathematically prohibited in classical physics. Demonstration of a realistic situation that violates the Bell inequality can confirm the existence of quantum entanglement, and thus also our understanding of quantum mechanics.
In 1972, John Clauser, from the United States, was the first to demonstrate a violation of the Bell inequality. He built a device that emits entangled photons - light particles - where each photon is emitted in a different direction. The property by which Clauser demonstrated this entanglement was light polarization; by measuring the polarization of the two entangled photons he showed that a violation of the Bell inequality had indeed taken place.
Clauser’s findings were not unequivocal, since the design of the experiment left theoretical situations in which transfer of information between probes that measure the polarization of each photon could occur without actual entanglement. This situation was solved by the French researcher, Alain Aspect, when he introduced some improvements into the experiment that ruled out any other way of interpreting the results, except for a violation of the Bell inequality.
The Austrian physicist Anton Zeilinger further refined the experiment, and among other things, demonstrated that it also works on three entangled particles instead of two, that it works at great distances and that it works even when further randomness is introduced into the system. His work also paved the way for quantum teleportation, which enables the transfer of information to great distances and even encryption of the information in unique ways.
Their work on quantum entanglement earned the three the Nobel Prize in Physics this year, after they shared the Wolf Prize in 2010 for the same contribution to quantum physics.
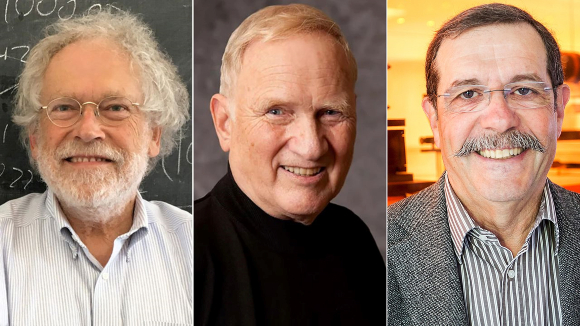
Gradual improvement of the original experiment. From left: Zeilinger, Clauser and Aspect | Photos: Jaqueline Godany, Peter Lyons, Ecole polytechnique Université Paris-Saclay, Wikipedia, CC BY-SA 4.0, 2.0
A Partial Trend of Improvement
Alongside the laureates of the Nobel Prizes in sciences, the Nobel Prize in Literature was awarded this year to the French author, Annie Ernaux, who has also written about her personal experiences with difficult issues such as Alzheimer’s disease of a loved one, in her book, “Remain in Darkness” and illegal abortions, in her book, “Cleaned Out”.
The Nobel Peace Prize was awarded this year to the human rights activist Ales Bialiatski, and to two human rights organizations - the “Center for Civil Liberties” in Ukraine and the “Memorial” organization in Russia. All three act in one way or another against the policies of the government in Moscow, which violates and undermines human rights in Russia and in the countries found under its influence.
The Nobel Prize in Economics was awarded to three American researchers. Ben Bernanke, who was the Chair of the Federal Reserve of the USA, will share the prize with the economists, Douglas Diamond and Philip Dybvig, for research that demonstrated the central role of banks during financial crises and helped to reduce the risk of economic crises with severe social consequences.
For many years, the Nobel Prize has been heavily criticized, sometimes justifiably and sometimes less so. One of the justified criticisms referred to discrimination against women scientists. To date, only 60 women have won the prize out of almost 1000 Nobel laureates, only six percent. In the sciences, the situation is particularly severe, with only 23 women laureates: four in physics, eight in chemistry and twelve in physiology or medicine (as well as only two in economics).
But in recent years there has definitely been an improvement in this trend. In the last four years the Nobel Prize in Physics has been awarded to two women, which is equal to the number of women Nobel laureates in the 117 years that preceded them. In chemistry, the number of women receiving the prize also doubled in the last four years, from four to eight. In physiology or medicine however, the trend has slowed down: six women have received the prize since the beginning of the 21st century, but no woman has received it in the last six years. It is to be hoped that the positive trend in the exact sciences will continue to strengthen, and that in the coming years we will see more equal treatment of women in all facets of life - both in academia and in the conferment of its most prestigious award.
