The instructors of almost every pastry course will turn to the students at some point in the first lesson, and say, in all seriousness, “Baking is an exact science.” Why do they say this? And what precisely do they mean? Maybe it really is science.
There are many similarities between baking and scientific experiments. Every time we make dough, cream, or frosting, we put exact amounts of materials in a bowl. The materials have to be added in a certain order and be subjected to such processes as stirring and heating in order for a new product, with different properties than those of its ingredients, to emerge.
In that sense, an experiment in chemistry is very much like baking. We add substances, or “reagents” as they’re called by chemists, in a certain order and in exact quantities into a vessel. The substances are subjected to processes that typically include mixing and heating, and ultimately, we get a new material that has very different qualities than the original ones.
In baking and experimenting, if we’re not making sure that we are using the precise quantities of the ingredients or adding them to the vessel in the prescribed order, the result will most probably be a failed experiment or pastry. Moreover, sometimes just mixing the materials too quickly or too slowly, or heating them to the wrong temperature, and once again, we end up with experimental failure or a scorched pastry.
So yes, there is similarity between science experiments and baking, but are the similar actions in the lab and kitchen the only connection between science and baking – or is there a deeper link?
List of ingredients
If we look at the different stages of baking as a chemist might, we’ll see that the sugar, butter, flour, and the rest of the ingredients are transformed into delicious cookies thanks to chemical processes. Each ingredient has a unique property and role that contribute to the final result and that is why it is so important to stick to the quantities, order of adding ingredients, and the baking process itself.
Chemistry in a cookie. TED-ED
Flour
Instead of looking at flour simply as a white powder, let’s consider its components and treat it as a source of starch and various proteins, mainly gluten. To make a dough that will give our pastry its basic structure, we initiate a chemical reaction in which the original materials are water and gluten. By mixing, kneading, and adding water, the gluten molecules can create a long, stable, and flexible net that forms the basis for the pastry.
Sugar
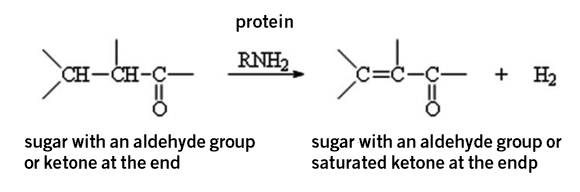
Sugar of course is a sweetener, but as you saw in the video, it also takes part in two important reactions: Caramelization, which is the partial decomposition reaction of sugars, and the Maillard reaction (which creates amazing scents and flavors) is a chemical reaction that takes place between proteins and sugars at high temperatures.
Baking powder
Baking powder is a mixture of baking soda and a weak acid. When the powder is exposed to water, a chemical reaction occurs that releases carbon dioxide as small bubbles, which confer volume and fluffiness.
|
|
As you can see, there are quite a few similarities between making a cake and creating a chemical compound in the lab. Moreover, the baker takes advantage of chemical reactions between the ingredients to create aromas, flavors, and structures that will make a sticky, flexible dough into a fabulous, fragrant, and tasty cake. However, it’s worth remembering that there are also differences: The type of materials, the type of reactions, the importance of controlling the work environment to avoid contamination, the attention to the level of purity of the original materials and byproducts, safety concerns, and more. If we go back to the beginning and to the question of whether baking is an exact science or not – well, you have all the necessary information to decide for yourselves.

