Orphaned in childhood, Dmitri Mendeleev overcame poverty and ailment on his path to finding a new method of organizing the chemical elements. Marking the 150th anniversary of the Periodic Table and 185 years since its discoverer’s birth
A number of chemical elements, such as copper, iron, silver, gold, and others were known to humanity for thousands of years, but the list of known elements remained largely unchanged since the days of the ancient Egyptian dynasties. This had changed in the mid-18th century, with the onset of a golden age in chemistry, when element after element was discovered – including oxygen, nitrogen, and chlorine. French chemist Antoine Lavoisier published a list of 33 elements in 1783, doubling the number of elements known only fifty years earlier. Within several decades, the number doubled once more, and by the middle of the 19th century, there were around 60 known elements.
Despite the dizzying pace of discovery, or perhaps because of it, utter chaos prevailed in the field of chemistry. Chemists were yet to fully understand what it meant for a substance to be an ‘element.’ For example, Lavoisier’s list included, in addition to the true elements, also heat and light (believed at the time to a substance termed caloric). Most scientists rejected the idea that matter was composed of atoms, and relied on Lavoisier’s simple rule, which stated that an element is a compound which cannot be separated into other compounds by a chemical reaction. Despite the confusion and the lack of clarity, the accumulating amount of information about the elements and their properties was so large, that someone had to organize it, discover the principles that would enable understanding the bonds between the various elements, and perhaps also shed light on the nature of the elements themselves.
Many tried to come up with a classification that would represent the elements’ different properties. Several scientists developed different arrangements which are more or less similar to the principles underlying the modern classification familiar to us today. When the fog of uncertainty cleared over this scientific breakthrough, one researcher stood above all the rest, whose name has become synonymous with the chart almost every high school student knows – the Periodic Table of Elements.
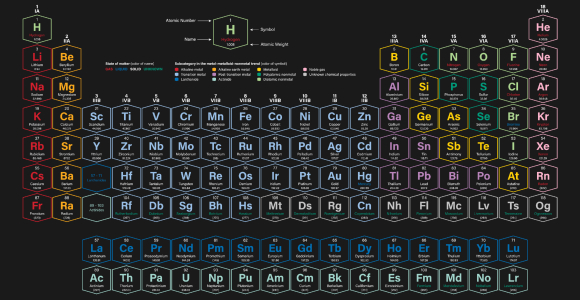
A scientific masterpiece representing many properties of the numerous individual elements. The Periodic Table of Elements. Source: Shutterstock.
Burnt glass
Born on February 8, 1834 in a small village near the city Tobolsk in Siberia, Dmitri Ivanovich Mendeleev (Менделе́ев) was the youngest of 14 siblings. His father was the principal of a local school who had lost his eyesight during Dmitri’s childhood and could not keep his job. To support the family, Dmitri’s mother, Maria, reopened the family’s glass factory. When Dmitri was 13 years old, his father passed away and the factory burned down. Most of Maria Dmitrievna’s grown children were already self-sufficient by then, and she decided to do all she could to provide her youngest child with the best possible education.
So the mother and son set off on horseback, riding thousands of kilometers westwards through the Ural Mountains to Moscow – but the prestigious University of Moscow refused to accept Mendeleev, as he was not a resident of the city. The two continued their journey, riding another several hundreds of kilometers to Saint Petersburg, then capital of Russia, where another disappointment awaited them: As a villager from Siberia without means, the local university would not grant him admittance. Eventually, Maria managed to enroll her son in the teachers’ academy where his father had also studied. She passed away several weeks later, and young Dmitri was left by himself, to fulfill his mother’s hopes and succeed in his studies.
An excellent student and a promising researcher, despite financial difficulties. Mendeleev in his twenties. Source: Science Photo Library.
Debts and books
Mendeleev indeed excelled, engaging in chemical research and publishing articles already during his studies. Contracting tuberculosis at the end of his studies, he travelled to work in the Crimean Peninsula, with the hope that the more agreeable climate would aid his recovery. After teaching at a high school for two years, he returned to Saint Petersburg, despite the fact that a permanent position did not await him there. He was steeped in debt and took on any job that came his way, school teaching, tutoring students in laboratories, giving private lessons, and even writing about research news in chemistry for a Russian education system journal. Two years later, he received a scholarship that enabled him to train in Paris and in Heidelberg, Germany, where he worked with the famous chemist Robert Bunsen and specialized in spectroscopy – a relatively new field of research. The field is based on the principle that each element reacts differently to light shined upon it, absorbing some wavelengths and emitting others. A methodological examination of a specific element at different wavelengths allows identification of its optical signature, and similarly, radiating an unknown compound reveals which elements it is composed of, based on the wavelength pattern it absorbs or emits.
When he returned to Saint Petersburg in 1861, Mendeleev was appointed as a lecturer in chemistry in the teacher’s academy. He was concerned about the low level of chemistry taught in Russia, as compared to what he had seen in Germany, and by the lack of good textbooks in Russian. At the age of 27, he composed, within a two month period, a comprehensive textbook in chemistry. Recognized by an important award (the Demidov Prize), the book won Mendeleev renown in his country. Of no less importance, the prize money enabled him to settle almost all of his debts. His fame helped him to secure a position as a lecturer at the University of Saint Petersburg, even before he finished his doctoral thesis. Only a year later, in 1865, he received his doctoral degree for his research on reactions between alcoholic compounds and water.
Mendeleev began working on another important textbook, ‘Principles of Chemistry’ (eventually published in 1869), and in parallel, began working on the scientific question that would occupy his mind in the following years – and earn him worldwide fame: A systematic order of the chemical elements.

A transition between versions – from Mendeleev’s early version to the modern periodic table. Illustration: Science Photo Library.
An irrational periodicity
During the 19th century, several researchers noticed that the atomic masses of different elements were actually whole multiples of the atomic mass of hydrogen. In other words, if the mass of hydrogen is taken as the basic unit, the mass of any other atom is a multiplication of a whole number of hydrogen units. The atomic mass of nitrogen is 14 times that of hydrogen, and gold – 197 times that of hydrogen, but there is no atom whose mass is 14.5 times that of hydrogen. At least, none were found with the measuring devices available to scientists then.
A German chemist named Johann Döbereiner noticed another phenomenon: Many elements can be divided into groups of three with a similar chemical behavior. Lithium, sodium, and potassium, for example, tend to react with oxygen at an identical ratio. When he organized these trios according to their atomic mass, he noticed that the atomic mass of the atom at the middle was approximately the average of the two others.
In Heidelberg Mendeleev had met the Italian chemist Stanislao Cannizzaro, who, in 1860, published a list of the atomic masses of the elements as ratios of the mass of hydrogen. Following this publication, additional scientists noticed the periodicity in the atomic masses of the elements, and even discovered a link between this periodicity and several other element properties, such as the specific weight (substance density), its boiling point, and its tendency to react with other substances.
Among the scientists who independently discovered this were the French geologist Alexandre-Émile Béguyer de Chancourtois and the British chemist John Newlands. de Chancourtois’s work was largely ignored, possibly because he was not able to explain his ideas clearly. Newlands, however, presented an interesting classification of the elements into eight groups, but the idea was considered ‘controversial’ by the Royal Society, at the time considered the most important scientific association, and it refused to publish his research. The Englishman William Odling also reached a similar chart, but his research was not published since he held an important position within the Royal Society, and there was suspicion that he had used his status to hinder Newland’s publication. The German chemist Gustavus Hinrichs took a different route and tried, without success, to develop an algebraic table of elements. A more successful table was created by another German, Luther Meyer, but, as he himself admitted, he dared not publish his work for fear of being ridiculed.
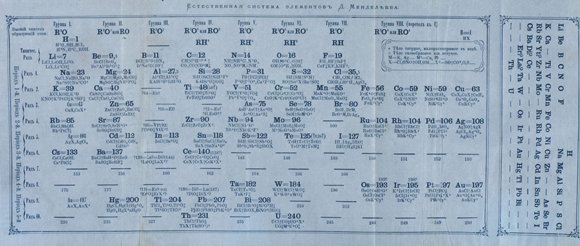
Inspired by a dream. A version of the table from 1871, with empty spaces and several predictions. Source: Science Photo Library.
From Dream to Reality
In contrast to several of his predecessors, Mendeleev as a chemist was highly experienced in lab work. Not only did he read about the elements in textbooks; he knew most of them from up close, which provided him with firm knowledge of their properties. In an attempt to match more easily the atomic mass and the elements’ properties, he created cards, each with the properties of one of the 63 known elements. Mendeleev played a game similar to solitaire with his cards, and tried arranging them in a way that made sense. A few days after his 35th birthday, he fell asleep on the table while playing with the cards. “In my dream, I saw a table where each element was in its proper place. When I awoke, I immediately wrote them down,” he would later write.
To realize the dream, Mendeleev understood that he had to implement some changes that other scientists rejected. For example, he switched between tellurium and iodine: Although tellurium is slightly heavier, he decided to replace it with iodine, immediately underneath bromine, with which it shares several properties.
According to this principle of similarity of properties between elements, in order to complete the arrangement of elements, Mendeleev had to leave empty spaces in the table – there were no elements whose properties fit those predicted by its neighbors. Bravely, or perhaps audaciously, he claimed that the missing elements will be discovered, even predicting their physical and chemical properties.
For example, Mendeleev predicted an element with similar properties to those of silicon but heavier, which would be placed below it in the periodic table. He called it eka-silicon, based on the Sanskrit word meaning ‘beyond’ or ‘after.’ Mendeleev predicted that eka-silicon will have the atomic mass of 72 (72 times the mass of hydrogen), and its specific weight would be around 5.5 grams per cubic centimeter. He also predicted that it would be a light metal with a relatively low boiling point, and that it could bind to two oxygen atoms, or alternatively, to four hydrogen or chlorine atoms. He predicted the future elements eka-aluminum and eka-boron.

The periodic table overshadowed his contribution to many other scientific fields. Mendeleev. Source: Science Photo Library.
The prediction is fulfilled
Mendeleev published his periodic table in 1869, and in another publication, in 1871, presented a more accurate table, in which he also included his predictions for the missing elements. Like every scientific innovation, Mendeleev’s table – and especially his predictions – were regarded with doubt by many other scientists, especially after so many had unsuccessfully tried to solve the challenge.
Within a few short years, public opinion shifted. In 1875, French chemist Paul-Émile Lecoq de Boisbaudran, engaged in research of zinc-rich minerals, identified an unknown element among them. He assumed that he had discovered a new element and began determining its properties, until the specifications seemed familiar to a colleague. A short search led to Mendeleev’s publication from four years earlier, and it was quickly revealed that the new element was none other than the eka-aluminum. The French researcher gave it a new name, gallium, in honor of his homeland’s previous name – Gaul. Four years later, the story repeated itself almost without deviation – this time, it was the discovery of eka-boron, named scandium by its Swedish inventor, Lars Nilson, for his native Scandinavia. In 1886, it was German chemist Clemens Winkler’s turn – he found an element matching the exact properties of eka-silicon, and he too named it in honor of his country – germanium.
It soon became clear that Mendeleev’s periodic table was the best way to organize chemical elements. Even elements whose discovery was preceded by the table found their place in it, and even when an entire group of elements were discovered – the noble gases – they all fell into place in a new column at the right of the periodic table. Years later, when science would evolve to create new man-made elements, they, too, were placed into the table based on their properties.
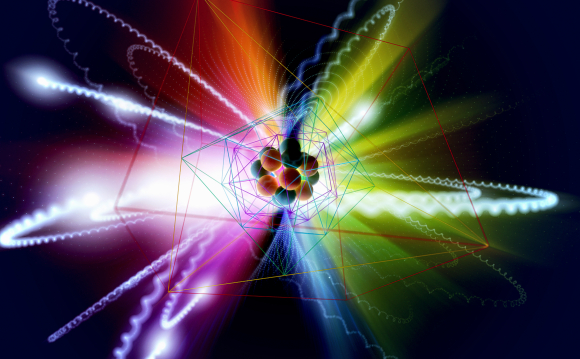
The arrangement of the protons, electrons, and neutrons is responsible for the organization of the periodic table. The structure of an atom. Source: Science Photo Library.
An internal organization
With the advance of chemistry and physics, and the understanding of the structure of the atom, it became clear why Mendeleev’s order was so successful, and its connection with the diversity of atomic properties became clear. Today, we know that an atom’s nucleus is composed of protons, which have a positive charge, and neutrons, almost identical, uncharged particles. The electrons orbiting the nucleus are negatively charge, thus balancing out the protons’ electrical charge, but their mass is so small it is practically negligible compared to the nucleus.
What differentiates between elements is the number of protons in their nuclei. The hydrogen nucleus contains a single proton, and therefore, its atomic weight is 1. The nitrogen nucleus contains seven protons and it also contains seven neutrons – thus, its atomic number is 7 and its atomic weight is 14 – like 14 hydrogen atoms. The nucleus of a gold atom holds 79 protons (and that is its atomic number), and 118 neutrons, therefore its atomic weight is 197.
The electrons are layered around the nucleus in shells, arranged somewhat like the layers of an onion. The innermost shell can hold up to two electrons, so the table’s top row contains only two elements – hydrogen, with its single electron, and helium, with two. The second shell can hold up to eight electrons, so the first element on the second row, lithium (atomic number: 3) has a full inner shell plus one electron in its second shell. Beryllium follows, with two electrons in the second shell, and so on, up to neon (atomic number: 10), in which both electron shells are full. The following rows are occupied by gradually heavier elements, with more and more shells.
The stability of atoms depends on whether or not their outermost shell is filled with electrons: If it is filled, the atom is stable. Elements in which the atoms’ outermost shell is unfilled can reach stability by taking or giving electrons, or by sharing electrons with other atoms. The number of electrons missing from the outer shell determines the chemical bonds that an atom can form, and thus, many of its chemical properties. Mendeleev arranged his table so that elements with the same number of electrons in the outer shell were in the same column, although he knew nothing about atomic structure. This property is of course the source of the Döbereiner triplets – lithium, sodium, and potassium all have a single electron in the outer shell, and so, react similarly.
A modern table of elements shows that the atomic weight of most elements is not a precise multiple of the weight of hydrogen, but usually a value close to it. This arises from the existence of isotopes – variants of an element differing only in the number of neutrons in their nuclei, and therefore, in their atomic weight. For example, about 99% of all carbon atoms in nature have the “regular” form, with six protons and six neutrons; there are few other isotopes, so carbon’s average atomic weight is 12.01. In contrast, only some 72% of all rubidium atoms in nature are the regular isotope (atomic weight: 85), and the remaining 28% are the heavier isotope (atomic weight: 87), so the average atomic weight of rubidium is nearly 85.5.
The structure of the atom and the bonds between atoms explain many other material properties, such as their states of matter, electrical conductivity, thermal conductivity, and so on.

Global renown. A Soviet postage stamp from 1969, marking the periodic table’s 100th anniversary | Source: Wikipedia, open use
A multidisciplinary scientist
The enormous success of the periodic table has made Mendeleev one of the most renowned scientists of his generation, but also overshadows his many other contributions to science. These include his involvement in petrochemistry – the study of oil and its products. He was among the founders of one of the first oil refineries in Russia, conducting research and development of fuels, fertilizers, and explosives. He also devoted his time to the study of the physical properties of materials and states of matter, and investigated as the connection between temperature and volume in different substances, the properties of solutions, and more. Some of his research paths were incorrect – for example, he mistakenly thought that ether may be another, lighter-than-hydrogen element, with the properties of a gas.
Beside his scientific activities, Mendeleev also engaged with numerous economic and social issues. He advised the Russian government on agriculture and trade agreements, and worked to protect the Russian economy through the introduction of import taxes. He was also one of the early advocates of women’s academic education and promoted the opening of lectures and courses to women.
The following anecdote illustrates Mendeleev’s eminent status in Russia. Upon divorcing his wife, he sought to promptly marry another woman (she was 19; he was 43), without waiting the several years required by the church, so he bribed a priest to perform the marriage ceremony immediately. When this was discovered, the priest was removed, but Mendeleev suffered no consequence. The official who complained about was told by the Czar, “Mendeleev indeed has two wives, but I have only one Mendeleev.” Whether or not this is true, in 1891, he was nonetheless forced to resign from the university because of his political support for extremist student groups.
He did not remain out of work, and shortly thereafter, was appointed head of the Bureau of Measurements and Weights – a standards institute of sorts. Another legend ties him to setting the standard for alcohol concentration in vodka, 40%, but as far as is known, there is no basis for this. Mendeleev also founded the Russian Chemical Society, and advanced the use of the metric system in the country.
He himself received numerous scientific awards in his lifetime, including the Royal Society’s Copley Medal and prestigious Davy Medal. In 1905 and again in 1906, he was nominated for the Nobel Prize, but lost in the close call. Time ran out on a third nomination: On February 2, 1907, he died of influenza, several days before his 73rd birthday. Ultimately, he received an even higher honor – element number 101 was named Mendelevium, immortalizing him in the periodic table he had developed himself.
A TED-Ed video on the genius of the periodic table:
