It is 65 years since Linus Pauling submitted his petition against nuclear testing, an adequate time to recall the story of one of the most important scientists of the 20th century
“Each nuclear bomb test spreads an added burden of radioactive elements over every part of the world. Each added amount of radiation causes damage to the health of human beings all over the world and causes damage to the pool of human germ plasm such as to lead to an increase in the number of seriously defective children that will be born in future generations. So long as these weapons are in the hands of only three powers, an agreement for their control is feasible”, wrote Linus Pauling in a petition signed by over 11 thousand scientists from around the world, which he submitted to the United Nations Secretary-General.
Pauling’s petition was just one of a long list of activities he led against the nuclear arms race, almost from its beginning, for which he was later awarded the Nobel Peace Prize. But even before that, Pauling was a very prominent figure in the scientific community, and is still considered one of the greatest chemists of the 20th century.

His activity earned him the Nobel Peace Prize. Pauling leading a demonstration against nuclear weapons testing | Photo: Science Photo Library
Bonds and Crystals
Linus Carl Pauling was born on February 28, 1901, in Portland, to Henry Pauling, a druggist, and his wife, Isabelle. By the age of 15 he had already completed the studies required to enter university, but due to technical reasons he only received his high school graduation certificate in 1962, after four decades of intensive research and a Nobel Prize in Chemistry.
In 1917 Pauling began studying chemical engineering at Oregon Agricultural College. He testified about himself that during his studies he read two papers that changed his life: “When I was 18, I read the papers of Irving Langmuir in the Journal of the American Chemical Society in 1919 and went back to Gilbert Newton Lewis’s 1916 paper. These papers, dealing with the nature of the chemical bond, the role of electrons in holding atoms together, interested me very much. That has been essentially the story of my life ever since”
In 1922, Pauling continued to a PhD in chemistry and mathematical physics at Caltech. His research, supervised by Roscoe Dickinson, focused on the use of X-rays to determine the atomic structure of crystals.
In parallel to his efforts on elucidating the atomic structure of crystals, Pauling’s interest in the nature of chemical bonds continued throughout his Ph.D., and he even met privately with Gilbert Lewis. The chemical bond was what occupied Pauling for the most part of his career, and his contribution in this field paved the way to him being awarded the Nobel Prize in Chemistry.
From Atom to Molecule
Ever since John Dalton laid the foundations for the modern atomic theory in 1808, chemists have tried to understand what causes atoms to bond to each other and to form molecules. In 1819, Jöns Jacob Berzelius suggested that a chemical bond forms as a result of electric attraction between two atoms with an opposite charge. However, Berzelius didn’t explain how bonds form between uncharged atoms, such as carbon, oxygen, or hydrogen.
In 1916 Lewis published a paper entitled The Atom and the Molecule, which Pauling read when he was 18. Lewis’ main claim was that a chemical bond forms when two atoms share electrons with each other. Moreover, a chemical bond will be stable if, as in noble gasses, after its formation each atom has eight electrons in its outer shell, a term Lewis learned from the atomic model of the Danish physicist, Niels Bohr, which was published a few years earlier.
After completing his Ph.D., Pauling received a two-year scholarship to study in Europe, where he conducted in-depth study of quantum mechanics, which was beginning to take shape during that period. He studied under the physicists who developed this new field, including Bohr, Erwin Schrödinger, and Arnold Sommerfeld.
One of the foundations of quantum mechanics is the uncertainty principle, according to which a particle, such as an electron, can, with different probabilities, be found at the same time in several different places. The mathematical equation developed by Schrödinger to describe this property is accurate but complicated. The Schrödinger equation can be solved for one electron, but as soon as one tries to use it to describe a more complex system - even two atoms that form together a simple molecule - it cannot provide an absolute solution.
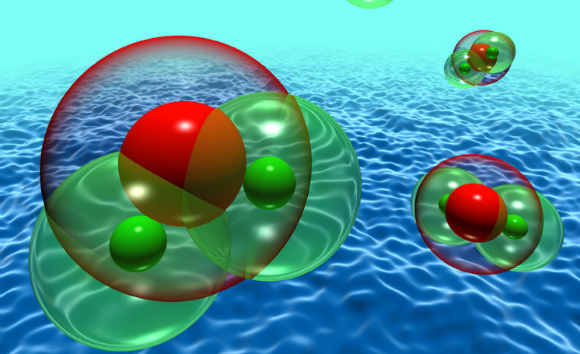
Pauling elucidated the nature of bonds between atoms. Illustration of the bonds between oxygen and hydrogen atoms in water molecules | Source: Science Photo Library
A Bond and a Half
Pauling realized, like others before him, that quantum mechanics is the key to understanding the chemical bond but also that its application to molecules is complicated. After returning to Caltech, he published a series of papers on the subject, which eventually became a popular book “The Nature of the Chemical Bond and the Structure of Molecules and Crystals”. In this series of papers, Pauling managed to eloquently simplify quantum mechanics with respect to its mathematical complexity and to derive rules that could be relatively easily applied to molecules.
One example of this is a phenomenon called “resonance”. If we return to Lewis’ terms, a single bond between atoms can be described as a bond between two electrons from two different atoms, and a double bond can be described as a bond involving four electrons. However, in some cases, such as in the benzene molecule, experiments have shown that the bond between two atoms is somewhere between a single and a double bond. Equipped with an insight from quantum mechanics, Pauling explained that there could be a state of “a bond and a half”, in which an electron spends half its time in one bond, and half its time in another bond.
After publishing the series of papers on the chemical bond, Pauling became interested in biochemistry and in the complex structures of DNA and proteins. In 1934 he began to study the magnetic properties of hemoglobin, the protein responsible for oxygen transport from the lungs to the rest of the body, and also worked on the properties of antibodies in the immune system.
During World War II, Pauling offered his services as a research consultant to the war effort, and made his laboratory available to the government. As part of these efforts, he developed, among other things, a device to measure oxygen concentration in the air, which was intended for military use in airplanes and submarines. Later on, the device was refitted for medical use, among other things, to monitor oxygen concentrations in NICU incubators. Nevertheless, he rejected Robert Oppenheimer’s offer to join the Manhattan Project to develop an atomic bomb, on the grounds that he didn’t want to uproot his family from their home.
Molecular Genetics
In 1945 Pauling heard a lecture about sickle-cell anemia, which causes a problem with oxygen transport in the body. After an in-depth research, he and his colleague, Harvey Itano, published a Science paper in 1949 entitled “Sickle-cell anemia, a molecular disease”, proposing that the cause of the disease is a change in one of the amino acids of hemoglobin. This change, known today as a genetic mutation, causes a change in the physical properties of hemoglobin and its ability to bind oxygen. Pauling’s discovery was published a few years before Francis Crick and James Watson elucidated the structure of DNA, which led to the realization that a mutation in DNA can lead to a change in the amino acid sequence and structure of a protein. Pauling was the first to propose that the disease is molecular in origin, and was even the first to use this term. Later on, Pauling’s assumption turned out to be correct, and today there are many diseases the molecular basis of which is known, serving as a starting point for drug development.
After a decade of focusing on biology, Pauling, who was experienced in elucidating the structure of mineral crystals using x-rays, managed to figure out the structure of a much more complex molecule - a protein. Using paper cut-outs, which he knew folded according to theoretical considerations, Pauling arrived at the exact structure of the alpha helix - a structure that can describe a particular section of a protein. Today, the atomic structure of hundreds of thousands of proteins has been published in the Protein Data Bank, and the alpha helix is an important component of many of them.
Pauling also tried to elucidate the structure of DNA, but originally assumed that it was a triple helix. Watson and Crick preceded him with their understanding of the structure of the double helix. “He was one of the founders who got the whole discipline going. And he got it going because… he understood chemistry and physical chemistry and he believed that that was the right way to think about these processes -- not in terms of mysterious forces”, said Francis Crick about Pauling’s contribution to the field.
Pauling also published important papers on many other topics in chemistry, including the magnetism of materials, the structure of metals and metal compounds, and the use of electron scattering to elucidate the structure of gas molecules. From 1940, Pauling’s name began to appear consistently in the list of candidates for the Nobel Prize in Chemistry. Fourteen years and sixty recommendations later, in 1954, he was awarded the prize for his research related to the understanding of the chemical bond and for his use of quantum mechanics to elucidate the ways in which atoms bond to one another.
War and Peace
With the end of World War II, Pauling, along with other scientists such as Albert Einstein, began to speak out against the spread of weapons of mass destruction. In the early 1950s, at the peak of the “McCarthyism” movement in the US, Pauling was considered by many almost as a traitor due to his statements, and was even interrogated by the Senate Committee for Immigration Affairs on suspicion of communist activity. Due to the suspicions, Pauling was denied a passport renewal and was forced to miss an important conference on the structure of proteins in London. Some argue that due to his absence from the conference he wasn’t exposed to important data about the structure of DNA, which was what allowed Watson and Crick to precede him.
After receiving the Nobel Prize in Chemistry, Pauling’s status improved, and he exploited this to expand his activity aimed at preventing the spread of nuclear weapons and to oppose the arms race between the United States and the Soviet Union. In his countless lectures he presented the public with alarming data that nuclear fallout from tests conducted outside the atmosphere increases the risk of cancer and the incidence of genetic diseases. At the height of his activity, on January 15th, 1958, Pauling and his wife Ava, who was most active in this advocacy effort, submitted a petition signed by about 11,000 scientists from around the world, which called for immediate cessation of nuclear tests, to the Secretary-General of the United Nations. The list of scientists who signed the petition included, among others, 2,848 scientists from the USA, 216 from the USSR, 1,161 from Japan, 150 from Germany, 57 from Israel, four from China and one from Lebanon.
Eventually, Pauling’s advocacy efforts contributed to a large extent to the signing of the Nuclear Test-Ban Treaty on July 25th, 1963, by the USA, the USSR and the United Kingdom. The treaty came into force at the end of that same year, and Pauling received the Nobel Peace Prize for his efforts. It is interesting to note that in 1962 the Prize Committee didn’t find a single deserving candidate; therefore, the prize Pauling received in 1963 was in fact for 1962.

Pauling obtained signatures from over 11 thousand scientists. A section of the petition in Pauling’s handwriting | Source: Pauling’s documents at the University of Oregon
Orange Season
Even after receiving the prize, Pauling continued his activism for peace. He spoke out against American involvement in Vietnam, Cuba, Iraq and other countries. Following pressure from conservative colleagues, for whom his anti-war efforts were like a thorn in their side, Pauling left Caltech in 1964, and served as a professor at San Diego University and Stanford University, until his retirement in 1973. During this time he broadened his interest in public health and in the influence of proper nutrition on disease prevention and life expectancy.
In 1970 Pauling published his book “Vitamin C and the Common Cold” in which he claimed that vitamin C can prevent colds. Later on, he even claimed that vitamin C could help in the treatment of cancer, heart disease, influenza and more. His claims regarding the contribution of vitamin C to curing cancer incited broad scientific and public debate, and subsequent studies demonstrated methodological problems in his research and cast a doubt on his conclusions. After his retirement from academia he was co-founder of a research institute, now named in his memory, which focuses on preventative medicine, with the understanding that proper nutrition can prevent many diseases and slow down the aging process.
Pauling passed away on August 19th, 1994, leaving behind four children and dozens of grandchildren and great-grandchildren. He is the only person to win two unshared Nobel Prizes, and many have described him as the most influential chemist since Antoine Lavoisier, who was one of the founders of modern chemistry in the 18th century. Pauling authored more than one thousand papers in a range of scientific fields and received dozens of awards. He left us a world with far fewer oranges, and hopefully one in which the notion that nuclear war is a bad idea is clear to all.
