In the following experiment, we'll use flammable materials found in citrus fruit peels to create a small flamethrower. This experiment requires adult supervision!
Equipment
- Thick-peeled citrus fruit, such as an orange, grapefruit or pomelo
- Peeling knife (use with adult supervision only)
- Candle
- Tray or dish for candle
- Lighter or matches (use with adult supervision only)
Instructions
Watch the video to see how to conduct this experiment:
If you have trouble peeling the citrus fruit properly, ask an adult to help you.
Explanation
Inside the cells of citrus fruit peels there are flammable oils and other carbon compounds, including alcohol. The oils give the citrus fruit peels their typical smells – the same smell that is left on our hands after peeling such a fruit.,These oils also protect the fruit from pests.
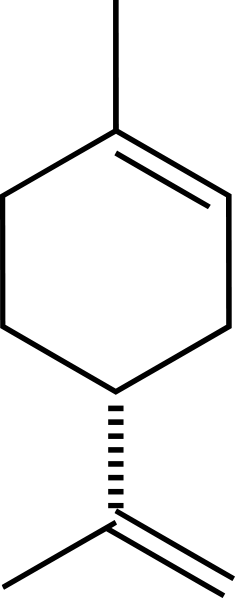
Structure of Limonene molecule, the main oil in the orange peel. Lines indicate chemical bonds between carbon atoms. | Credit: Wikipedia
When you press the peel, the cells are squeezed and the oil is pushed out. When these flammable oils hit the flame, they burn and create an amazing effect, as can be seen in the film. It's like a small military flamethrower, except that real flamethrowers work with petroleum-based fuels.
Additional Information
The aromatic oils in citrus fruit peels are mostly used in the perfume and food industries to give a citrusy smell. Recently, medical uses have been found for these oils as well, including an asthma medication from orange peels, and anti-bacterial and anti-fungal materials derived from grapefruit peels.
Dr. Avi Saig
Department of Neurobiology and Davidson Institute of Science Education
Weizmann Institute of Science
Article translated from Hebrew by Aviv J. Sharon, M.Sc. student at the Weizmann Institute of Science.
Note for Surfers
If you find the explanations unclear or have further questions, please drop us a line on the forum. We welcome your comments, suggestions and feedback.
