Why does hepatitis lead to the yellowing of skin and eyes in patients? How do the hepatitis-causing viruses differ from each other and what are the risks associated with each type? What treatments and vaccines are currently available to fight hepatitis?
The liver is an essential organ. Its many functions include breakdown and neutralization of toxins, regulation of substances that move from the digestive system to the bloodstream, and production of the bile salts required for fat metabolism. Hepatitis, or liver inflammation, is a disease of the digestive system resulting from damage to the normal functioning of the liver.
One of the identifiable symptoms of hepatitis is jaundice, that is, the development of a yellow hue in the skin and eyes. This color results from the accumulation of a substance called bilirubin in the body’s tissues. Bilirubin is a byproduct of the breakdown of red blood cells that have completed their life cycle, a process that typically occurs in the spleen. Under normal circumstances, bilirubin is efficiently metabolized by the liver and is excreted from the body through feces. However, when the liver’s function is impaired, bilirubin remains in the bloodstream, causing the skin and eyes to turn yellow.
An excess of bilirubin in the bloodstream is also characteristic of neonatal jaundice, a phenomenon observed in about 40% of newborns. This type of jaundice is not caused by inflammation or liver damage, but rather by a large amount of red blood cells in newborns, and is nearly always treated easily, without any adverse effects to the infant.
Most cases of jaundice that are not neonatal jaundice result from infectious hepatitis, subsequent to an infection by a virus that attacks the liver tissue. The most prevalent pathogens causing infectious hepatitis are five types of hepatitis viruses, categorized as A, B, C, D, and E. These viruses belong to distinct families; but they share a tendency to infect liver cells and induce inflammation. Every year, viral hepatitis claims the lives of approximately 1.1 million people worldwide.
Hepatitis A and Hepatitis E viruses, which cause acute disease, are typically transmitted from person to person through contaminated food or water, due to poor sanitation and inadequate hygiene. Hepatitis B and Hepatitis C viruses are predominantly transmitted through bodily fluids. They can also cause severe illness, but it is a chronic disease, potentially lasting for many years. In rare cases, the Hepatitis E virus can also lead to chronic illness. To date, about 350 million people worldwide are infected with one of these viruses. The Hepatitis D (delta) virus can only infect individuals that have already contracted hepatitis B and it is one of the factors contributing to the worsening of liver disease in chronic patients.
The symptoms associated with all of these viruses tend to be similar; they include a high fever, general malaise, loss of appetite, abdominal pain, dark urine, and as mentioned earlier - jaundice. In order to accurately identify the virus responsible for the illness and determine the appropriate treatment for the patient, it is necessary to perform a test to identify the genetic material of the virus or the unique antibodies produced by the patient’s immune system in response to the infection.
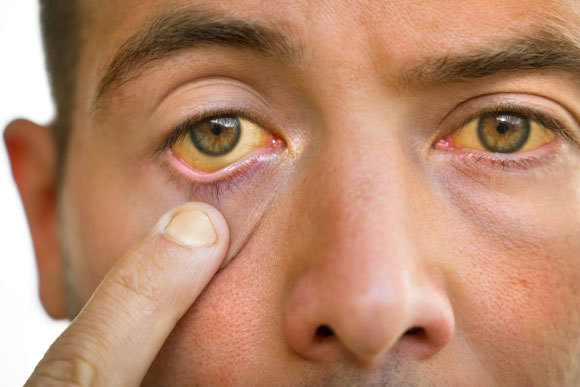
The yellow hue of the skin and eyes is a result of the buildup of a substance called bilirubin in the body’s tissues. An individuals demonstrates his yellow eyes, a symptom of liver disease | Shutterstock, Creative Cat Studio
Viral Hepatitis A
Hepatitis A virus (HAV) is an RNA virus from the Picornaviridae family, which also includes the polio virus, as well as viruses causing the common cold, among others. This virus is excreted through feces and is transmitted by either consuming contaminated food or via close contact with an infected individual. Hepatitis A is the most prevalent type of viral hepatitis.
In children up to the age of five, the disease can potentially manifest without any symptoms whatsoever. Adults, on the other hand, typically report high fever, weakness, nausea and vomiting, joint and muscle pain, along with occasional flu-like symptoms. Jaundice, characterized by dark urine and pale feces, typically appears one to two weeks after the disease onset. At this stage, other disease symptoms typically diminish and the main symptoms remaining are a feeling of weakness and loss of appetite.
Although the virus causes a severe illness, it typically resolves within one to two months and does not develop into chronic hepatitis. In rare cases it can develop into a severe disease characterized by severe liver failure, which might necessitate an urgent liver transplant. This risk predominantly applies for older patients with existing health conditions.
Today, Hepatitis A infection can be prevented through vaccination. In the United States, the Centers for Disease Control and Prevention (CDC) recommends Hepatitis A vaccination for all children. The two main vaccines primarily in use are the active HAVRIX and VAQTA vaccines. Both contain inactivated (“killed”) Hepatitis A virus and are supplemented with an adjuvant - a substance that enhances the immune response
The vaccine is administered to all children from the age of one, in two doses. It is also recommended for patients with chronic liver disease, HIV carriers, individuals exposed to hepatitis A patients, and travelers visiting countries where the virus is prevalent. The effectiveness of the vaccine in preventing the disease is estimated to be around 94% and it remains valid for approximately 20 years. Since its inclusion in the routine vaccination schedule in the country, the incidence of Hepatitis A has decreased by about 90%.

Hepatitis A is the most common type of viral hepatitis. Electron microscope image of the virus causing the disease | James Cavallini, Science Photo Library
Viral Hepatitis E
Hepatitis E virus (HEV) is also an RNA virus, but it belongs to the Hepeviridae family. This virus is typically excreted in feces, and infections often occur due to oral ingestion of contaminated water. Hepatitis E is primarily prevalent in Southeast and Central Asia, North Africa and Central America.
The course of Hepatitis E disease mirrors that of Hepatitis A, exhibiting similar symptoms. In most cases the inflammation resolves naturally and does not lead to a chronic condition. However, some immunocompromised individuals face difficulty in overcoming the virus. Hepatitis E is also known to pose a significant risk to pregnant women, and may lead to severe symptoms and even death.
Unlike Hepatitis A, scientists have not yet been successful in growing hepatitis E viruses in culture under laboratory conditions, thus it has not been possible to develop a vaccine based on an inactivated virus. As a result, vaccine developers have primarily concentrated their efforts on genetic engineering methods. These involve presenting the envelope proteins of the Hepatitis E virus to the immune system in order to stimulate the production of antibodies against them.
To date, the only approved vaccine against hepatitis E is licensed, manufactured and marketed exclusively in China and is approved for use in individuals aged 16 and above. Distribution of the vaccine in China began in 2011 and data from 2014 indicates that it reduced the incidence of the virus by only 7.5%. The vaccine has yet to be tested on high-risk populations, such as pregnant women and immunocompromised individuals. Consequently, these sensitive populations are not eligible to receive the vaccine, and its effectiveness in preventing incidence and death particularly in these groups remains uncertain.
Despite the great need for an effective vaccine against viral hepatitis E, particularly in outbreak-prone regions, it seems that extending its distribution to other countries outside of China poses significant challenges. First, the current vaccine requires three doses at specific intervals of one month and six months from the initial dose, making it less effective for rapidly controlling local outbreaks of the virus in unvaccinated populations. Secondly, there are four slightly different variants of the virus worldwide, each with slightly different envelope proteins. The Chinese vaccine is designed to target the envelope proteins of the virus variant prevalent in China (variant 1), but its protective efficacy against other Hepatitis E virus variants remains unclear. Furthermore, the vaccine has yet to receive approval from the World Health Organization (WHO). Therefore, in the meantime its use will not extend beyond China.
Viral Hepatitis B
Hepatitis B virus (HBV) is a DNA virus from the Hepadnaviridae family. It is transmitted from person to person by exposure to infected blood, through sexual contact, and from an infected mother to her newborn.
The disease develops slowly. After an incubation period that lasts from one to six months, a preliminary stage characterized by high fever, joint pain, and rash, develops in 10–20 percent of patients, followed by acute hepatitis, which can last between one and three months. Jaundice develops in only about 30 percent of cases, with others experiencing mild symptoms to no symptoms at all. Symptoms may include high fever, nausea, loss of appetite, upper right abdominal pain, and a disruption in liver function detectable by a blood test.
Most cases of Hepatitis B generally resolve within one to three months, during which patients receive only supportive care. However, a subset of patients may develop chronic disease. The risk of progressing to chronic disease decreases with age: while adults face a 4 percent risk of chronic disease, nine out of ten newborns infected with the virus will develop chronic disease.
Chronic disease may develop in some patients infected with Hepatitis B. Electron microscope image of Hepatitis B virions | Credit: James Cavallini, Science Photo Library
In chronic cases of Hepatitis B, the patient’s immune system is unable to get rid of the virus completely, which survives and continues to replicate within liver cells. Complications often take years to develop and may include liver cirrhosis - a condition in which functioning liver tissue is destroyed and replaced by non-functioning scar tissue - and even liver cancer. In Israel there are approximately 200,000 carriers of the virus, of whom 5–10% have chronic disease. In the United States, up to 1.25 million individuals are HBV (hepatitis B virus) carriers and up to 2.4 million are chronically infected.
Treatment for these chronically ill patients may include interferon treatment, which stimulates the immune system to combat the virus. Additionally, broad-spectrum antiviral medications can be employed. These drugs have a similar enough structure to the genetic building blocks of the virus that they can be integrated into it, but are different enough to prevent virus replication and proliferation. These medications significantly reduce the amount of virus in the body, allowing the immune system a better chance at overcoming the disease. Nevertheless, these drugs can have side effects, and the virus may develop resistance to them over time.
The first vaccine against Hepatitis B virus (HBV) was developed in the 1970s. The vaccine relied on isolating viruses from the blood of hepatitis patients and neutralizing them so that they couldn’t infect those receiving the vaccine. The production process was complicated and carried risks since it was based on the blood of hepatitis patients. This method was abandoned once safer and more effective vaccines were developed.
In 1986, a genetically-engineered vaccine was introduced. This vaccine prompted non-harmful cells, such as yeast cells, to present one of the virus’s envelope proteins to the immune system, along with added aluminum salts intended to induce an immune response. The vaccine is given in three doses and provides 94–98% protection from chronic disease for about 20 years. Since 1992, all newborns in Israel have been vaccinated with an active vaccine based on this technology.
While the Hepatitis B vaccine is effective and safe, it doesn’t provide full protection for certain high-risk populations. Its efficacy is notably lower in protecting the elderly, smokers, overweight individuals, and those with kidney disease.
In 2018, the U.S. Food and Drug Administration (FDA) approved an improved vaccine against HBV. The new vaccine utilizes the same envelope protein as its predecessor, but includes a different adjuvant to stimulate a stronger immune response, even in populations that developed a weaker immune response to the previous generation of vaccines. This advantage is particularly valuable for protecting at-risk populations, such as the elderly with underlying conditions. Another benefit of the new vaccine is its two-dose regimen, as opposed to the previous three-dose protocol, which may improve the responsiveness to receiving the vaccine and make it easier to administer.
Recently, the FDA approved a new-generation Hepatitis B vaccine for distribution in the U.S. This new compound, based on the research of Prof. Yosef Shaul from the Department of Molecular Genetics at the Weizmann Institute of Science, incorporates three of the envelope proteins of the virus rather than just one. Thus, it provides broader protection and it has indeed been found effective in protecting groups that were insufficiently protected by the standard vaccine. The vaccine, having successfully passed safety and efficacy trials, is now in use in 14 countries, including Israel. The European Medicines Agency (EMA) has also recommended incorporating this vaccine into routine immunizations across European Union member states.
Recently, the FDA approved the distribution in the US of a new generation vaccine against the type B hepatitis virus. A vaccine bottle and syringe | Shutterstock, ronstik
Viral Hepatitis C
Hepatitis C virus (HCV) is an RNA virus from the Flaviviridae family, which also includes the viruses that cause Zika fever and Dengue fever, among others. Its primary mode of transmission is through exposure to infected blood and unprotected sexual contact. The incubation period typically lasts between two weeks and three months, and may be followed by hepatitis symptoms lasting 4–6 weeks. However, at this stage of the disease, most patients will not experience symptoms.
Only about 30% of patients fully recover, while most others develop chronic disease characterized by recurrent flare episodes with intermittent breaks, with the virus continuing to replicate and damage liver cells. Over time, the damage inflicted to the liver tissue can lead to liver cirrhosis in 15–30 percent of patients and an increased risk of liver cancer.
Historically, drug treatment of the disease was not particularly effective, comprising weekly injections of interferon, which, as mentioned, stimulates the immune response, along with broad-spectrum antiviral drugs. This treatment only led to the recovery of roughly half of the patients, while for the rest it was mainly effective in suppressing flare episodes.
Substantial effort has led to the development of excellent drug treatments against the virus that lead to full recovery. They are given in pill form and their side effects are minor. The treatment lasts 8–24 weeks and targets specific components in the virus, inhibiting its replication. It combines several drugs directed at separate targets, to prevent the virus from developing resistance to the drugs. For example, the drug Mavyret contains a combination of two active ingredients: one of which inhibits the activity of a protein that processes the viral proteins into their final shape, and one that inhibits a protein necessary for replication of the virus’s genetic material.
A significant drawback of these drugs is their high cost, ranging from USD 20,000–100,000 per treatment. Thus, the treatment is approved in Israel only for carriers of the virus suffering from liver cirrhosis marked by significant scarring of the organ’s tissues. Patients whose state of health is less severe must either wait or pay for the drugs out of their own pocket.
Currently, there is no vaccine against this virus. One of the main challenges impeding the development of a vaccine is the diversity of the virus’s forms and characteristics: the Hepatitis C virus has eight different main variants and about ninety sub-variants differing from each other in their envelope proteins. Since the genetic material of this virus is RNA, which lacks mechanisms for error correction during its replication, it rapidly accumulates more and more changes, making it very challenging to find a suitable protein against which to develop a vaccine.
Only about 30% of patients will make a complete recovery, with most of the others developing chronic disease. Electron microscope image of Hepatitis C Virus (HBC) | Credit: James Cavallini Science Photo Library
Viral Hepatitis D
Hepatitis D Virus (HDV) is an RNA virus from the Kolmioviridae family. It shares the same transmission routes as previously mentioned viruses: infected blood and sexual contact.
This virus stands out among hepatitis viruses due to its inability to survive independently - it requires the aid of another virus. This is due to its lack of appropriate envelope proteins, which would enable it to bind to receptors present on the surface of liver cells, allowing it to penetrate these cells. Therefore, when present in the human body, it necessitates the presence of Hepatitis B envelope proteins in order to infect cells and propagate.
Roughly 5% of Hepatitis B patients also carry the Hepatitis D virus. Typically, such a dual infection is more severe than an infection with Hepatitis B alone, and is characterized by a more severe disease the complications of which may lead to liver failure, liver cirrhosis, or liver cancer.
Treatment of patients with Hepatitis D is usually similar to the treatment given to Hepatitis B patients, and includes interferon and broad-spectrum antiviral drugs. The most effective prevention is vaccination against Hepatitis B, which also inhibits the infection and spread of Hepatitis D.
Despite the continued toll of hepatitis viruses, the development of medications and vaccines, together with improved access to clean sources of water and maintenance of hygiene have markedly reduced the incidence of viral hepatitis and the resulting mortality. In 2016 the World Health Organization launched a work plan for eradicating Hepatitis B and Hepatitis C by 2030. The path to achieving this objective still appears long, but continuous advancements in vaccine technology and the development of potent antiviral medications are decidedly bringing us closer to this goal.
