Beyond gold: Some metals cost tens of thousands of dollars per kilogram, and radioactive elements fetch even more. Would you break your piggy bank to buy a gram of plutonium?
The many elements in the periodic table differ in prevalence, difficulty of finding, and price. Oxygen (O) and nitrogen (N) are available more or less for free in the air we breathe; carbon (C), aluminum (Al), and others are inexpensive and common; but certain elements are particularly expensive, while others cannot be found in nature or produced in large quantities. Meet these precious elements – and find out why they are so pricey.
Form first
Some elements considered to be precious actually come quite cheaply. Silver (Ag), for example, costs around 550$ a kilogram – cheap and cheerful in comparison to some of the prices we’ll see later on. One of the cheapest elements in the periodic table, by the way, is iron (Fe), with a price tag of 100$ per ton – i.e., well under a dollar per kilo. Sulfur (S) is similarly quite cheap.
Then there are elements which are expensive only in some of their forms. Diamonds are the obvious example: A very rare and expensive form of carbon, where the atoms are arranged very differently from the element’s more common forms – coal and graphite. Natural diamonds that are flawless and of the highest quality can fetch tens of thousands of dollars per gram in price.
Radioactive isotopes of elements that have stable isotopes are another example. Isotopes are forms of an element that contain an equal number of protons but a different number of neutrons in the nuclei. While this difference typically does not affect the element’s chemical properties, it can affect the nuclear properties (e.g., radioactivity), and physical characteristics (density, for example).
Tritium (T or H3), for instance, is a useful radioactive isotope of hydrogen that is rare and expensive, while hydrogen itself is usually a cheap element. This scarcity means that tritium costs around 30,000$ per gram. Of course, most of its applications require a small quantity.

|Tritium: A different form of one of the world’s most common elements can cost 30,000 per gram. Photo: Teravolt, Wikipedia.
Additional examples of elements considered precious only in certain forms are boron (B) and fluorine (F). Both elements are cheap and quite common as part of compounds – but rather expensive in their pure state. Boron is very difficult to produce without impurities, while fluorine is extremely reactive; even storing it so that it doesn’t corrode its container is a challenging mission. Both cost several thousands of dollars per kilogram if you need them as pure elements.
Cesium (Cs) and rubidium (Rb) are highly active metals and occur naturally only as salts – compounds of the metal’s ions and other ions. These salts are relatively cheap, but if you seek them in their pure metal states, you would have to pay tens of thousands of dollars per kilogram as it is so hard to extract from their salts.

Cheap when in compounds, costly when pure. Fluorine (right) and boron crystals. Photos: Science Photo Library.
Not all that glitters is gold
The other expensive elements in the periodic table are metals, whose cost runs upwards of thousands of dollars per kilogram.
The first group of elements are metals from the platinum family: Ruthenium (Ru), rhodium (Rh), palladium (Pd), osmium (Os), iridium (Ir), and platinum itself (Pt). These elements share some similarities, usually co-occur in nature – but always in very small quantities, and they are also very hard to isolate, so that mining them is not profitable. They are thus produced only as mining byproducts of other metals, chiefly nickel (Ni).
And they are priced accordingly. Except for ruthenium, these elements cost over 10,000 dollars a kilogram, typically well over. Rhodium is the most expensive, even though osmium is rarer – in fact, it is the rarest stable element in Earth's crust: Less than 1,000 kilograms of it are produced annually, so substitutes are generally preferred, and the limited demand, in turn, keeps the price down. Ruthenium is relatively cheap – but only when compared to the others in this group; its price is still several thousands of dollars per kilogram.
All of these elements are relatively chemically unresponsive – they do not readily react with other elements and are therefore barely affected by oxidation or corrosion (for example, the rusting of steel when it comes into contact with oxygen). This property contributes to their considerable price tag and uses. For example, the standard meter and standard kilogram used to define these units are made of an alloy of platinum and iridium.
Platinum is used mainly for jewelry making and is sometimes referred to as “white gold”, but it also has many industrial and medical applications. The other elements in this group have mostly industrial uses, for example, as catalysts in catalytic converters which reduce the emitted pollution in cars.
Another expensive metal is gold (Au), of course. It is one of the least chemically reactive elements and is expensive, but its chemical properties are different from those of the metals in the platinum group and it does not occur naturally with them.
The price of gold is around 52,000$ per kilogram, less than platinum or rhodium but more than other metals in the platinum group, depending on market fluctuations. Rhenium (Re) is also a rare and expensive metal, yet its price is lower, in the range of several thousand dollars per kilogram.

White gold: Platinum is used not just for jewelry, but also in medicine and industry. Photo: Science Photo Library.
Hard to come by
Another group of precious metals that are rare in nature or are difficult to produce are found at very low concentrations, together with elements of similar properties, so that their extraction from ores is no simple matter.
This group includes scandium (Sc) which has similar properties to aluminum. It is quite chemically active and is not particularly rare – but the difficulty in finding and extracting it stems from the fact that it is not concentrated in large amounts anywhere, hiking its price to over 10,000$ per kilogram.
Thallium (Tl) is a toxic element, as the body has difficulty in distinguishing it from potassium, which is vital for many of our biological functions. It is very expensive because it is both difficult to produce and is very rare, driving its price to several thousands of dollars per kilogram. The lanthanides (a group of metals with the atomic numbers 57-71) lutetium (Lu) and thulium (Tm) also cost around 10,000$. Lutetium was once harder to produce and therefore very expensive. It was the last lanthanide to be found in nature, and one of the last stable elements to be discovered. As their production is not financially viable, they are expensive and produced only as by-products of other metals.
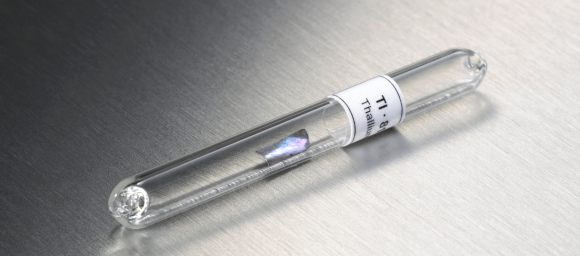
Rare, hard to produce, toxic, and costly, at thousands of dollars per kilogram – a tiny piece of thallium. Photo: Science Photo Library.
Caution: Radiation
The third group of precious metals are the radioactive materials. There are several naturally occurring radioactive elements, but among them, only uranium (U) and thorium (Th) exist in large quantities and so are relatively inexpensive. With the exception of plutonium, all other radioactive elements cannot be obtained in quantities of over a few kilograms. For most of these elements, the total quantity ever produced is no more than a few grams.
Some of these elements are simply incredibly rare and occur in tiny quantities, for instance, actinium (Ac) and astatine (At). Others, such as technetium (Tc) and neptunium (Np), can actually be produced in significant quantities in nuclear reactors, but are typically not separated from the nuclear waste. And some elements simply do not occur in nature at all and are only produced in small amounts in nuclear reactors. For example, the total quantity ever produced of americium (Am) and curium (Cm) is just a few kilograms; and even less was produced of the heavier elements.
Most of the world’s nuclear reactors generate electricity, and any heavier-than-plutonium element produced in them is usually a byproduct – sometimes a wanted one – of the reactor’s operation. The distribution of these elements is restricted and only authorized laboratories can obtain them. So even if you were in dire need of plutonium for your DeLorean time machine, you would not be able to buy it legally in the U.S. or in Israel.
And if you were to establish a facility equipped for and authorized to handle radioactive materials, such as a research or a military institute, you would still need to pay. How much? A lot!
Nuclear weapons-related plutonium production requires a reactor and other facilities dedicated to plutonium separation. That is why weapons-grade plutonium probably costs hundreds of dollars per gram, if not more. That is,. hundreds of thousands of dollars per kilogram – and ten(or more) times more expensive than gold. Making a nuclear bomb requires at least four kilograms of plutonium, the raw materials for such weapons cost over a million dollars. And there is no market for this material, so that this is just the production cost.
Weapons-grade enriched uranium, of which uranium-235 comprises at least 93%, , is much cheaper, though twice as expensive as gold – around 100,000$ per kilogram. Once again, this is the production cost, as the material is under strict control, and a private person or commercial entity cannot obtain it freely.
When it comes to other radioactive elements, there is no point in pricing them per kilogram, since their quantities ever produced amount to a few kilograms in total. The cost of curium and americium, which have commercial use in smoke detectors, is similar to plutonium, even a little higher: Curium is priced at several hundreds of dollars per gram, while americium is about 1,500$ per gram. But since smoke detectors need less than a millionth of a gram of americium, this is actually quite affordable.
Californium (Cf) is the heaviest element with industrial applications outside of the laboratory – and it is staggeringly expensive: One millionth of a gram is priced in the dozens of dollars, so that one gram costs tens of millions of dollars. However, the total quantity that has ever been produced is just a few grams. All of its uses are based on its strong neutron emission as a result of its radioactivity, and thus required quantities are tiny. In the past, annual worldwide consumption reached 150 milligrams a year, which means that these applications are not too expensive.
Berkelium (Bk), the last element heavier than uranium that can be produced in relatively large quantities, of tens or hundreds of milligrams, has virtually no use outside the lab. It is even more expensive than californium – around 200$ per one millionth of gram, around five million times more expensive than gold.
The only lighter-than-uranium radioactive element with a relatively broad range of applications outside of the lab is polonium (Po), in its isotope form polonium 210. It costs hundreds, if not thousands, of times more than gold. Its use is restricted to minute quantities of just a few milligrams. Global annual production is relatively sizable for this synthetic element – about 100 grams a year are actively produced; it is not a byproduct of nuclear reactors, but rather requires a dedicated process. Polonium 210 is particularly infamous for its role in the assassination of Russian dissident Alexander Litvinenko in London in 2006.
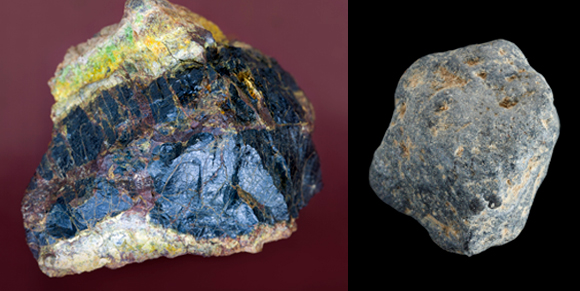
Uranium-rich ore (left) and thorium. Relatively cheap materials, but subject to tight supervision. Photos: Science Photo Library.
The alchemists
The alchemists of the Middle Ages dreamed of turning lead into gold. Can we now manufacture rare and precious elements from cheaper ones, just like heavier-than-uranium elements are created in nuclear reactors? While turning lead into gold isn’t really lucrative, most precious metals can be synthesized in the lab from other elements, at least in theory.
There are two possible ways to do this: The first is based on the fact that the platinum group’s light metals – ruthenium, rhodium, and palladium – are also products of nuclear fission, and therefore can be found in nuclear waste. In other words, nuclear fission of uranium can be considered as a way to turn the inexpensive uranium into precious metals. This method has been examined in the past, as was the production of silver, which is also generated in nuclear fission. However, the difficulty in disposing of the products’ radioactive contamination and the lofty price of removing the highly radioactive waste renders this process economically unviable.
The second method is based on radiating an element with neutrons in a nuclear reactor to turn it into another element, usually one or two places away in the periodic table. In this method, one of the neutrons absorbed in the nucleus becomes a proton (by emitting an electron), making the element heavier. The problem is that the precious elements, as we have seen, tend to be close to one another; therefore, using this method to produce a precious element requires starting with an element that, while cheaper, is nevertheless precious in itself. For example, the production of gold requires starting with platinum, which is very expensive in its own right, or mercury – which would mostly create radioactive isotopes of gold.
Other possibilities considered in the past include converting tungsten (W) to rhenium (Re), rhenium to osmium (Os), osmium to iridium (Ir), and platinum to iridium, as well as ruthenium to rhodium. All of these have never been commercially executed and such a process does not appear to be financially viable in the foreseeable future. The reasons are similar: The method is quite expensive and can produce radioactive pollution in the product, which, in turn, will greatly reduce its value.
Perhaps someday we will perfect the technique to produce precious metals from cheaper metals, or, in other words, become alchemists!
