Potatoes break down in our bodies into… sugar! How does this happen? A visit with the carbohydrate family
Like all of the other carbohydrates we eat, potatoes break down into sugars inside our bodies. How does that process work? What does the final product look like? Are carbohydrates healthy? Watch the video clip:
We Are Family
Carbohydrates are a vast family of substances present throughout the animal and plant kingdoms. Their name is derived from their chemical formulae—a combination of the words “carbon” and “hydr-” (stem of the Greek word for water). Their chemical formula looks something like this: Cm(H2O)n. In other words, carbohydrates contain a certain number of carbon atoms (chemical symbol—C) and a certain number of water molecules (H2O). Let’s look at the formula for glucose, mentioned in the video clip: C6H12O6, which can also be written as a combination of six carbon atoms with six water molecules: C6(H2O)6. Similarly, the formula for sucrose—a common food and drink sweetener—can be written as a combination of 12 carbon atoms and 11 water molecules: C12(H2O)11. The general formula for carbohydrates fits most of the “family” members, although with time, exceptions have emerged, but the name was kept. Sugar is “a carbohydrate that tastes sweet,” thus all sugars are carbohydrates, and in practice, the terms carbohydrate and sugar are used interchangeably.
Mono, Di, Poly
The members of the family can be divided into three general groups, in an ascending order of complexity: Monosaccharides, disaccharides, and polysaccharides. In the video clip, I start with the starch, a polysaccharide found in potatoes and break it down, first to the disaccharide maltose, and ultimately to the simple, common monosaccharide, glucose. This direction is the opposite that of their formation process, which goes from simple to complex. To explain, we’ll begin at the beginning, or in other words, with the monosaccharides.
Monosaccharides are the building blocks of all carbohydrates, and glucose (derived from the Greek word for “sweet wine”) is actually the starting point for the entire living world. Glucose is the final product of the photosynthesis process in plants. In this process, plants use sunlight to convert water and carbon dioxide into glucose and oxygen. This process converts inorganic materials into organic compounds. As the sole organic compound synthesized in this process, glucose is the origin of all the other compounds; that is, it is the starting point for all other organic compounds—the compounds of the living world—which explains its tremendous importance to living organisms. Every carbon atom in every animal originates in a glucose molecule. As mentioned earlier, glucose has six atoms of carbon, each atom linked to (at least) one oxygen atom and one hydrogen atom. The glucose molecule tends to behave like a “snake eating its tail”—with one end of the molecule connecting to its other end, creating a hexagonal ring. We can draw the molecular structure of glucose as follows:
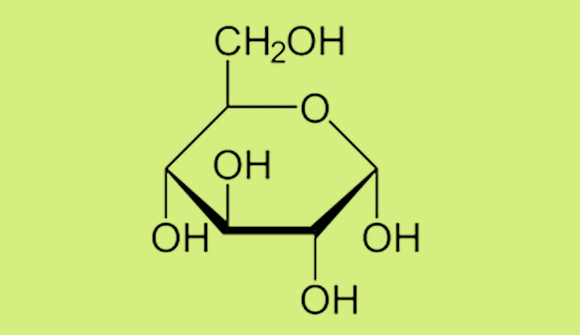
A Haworth projection of the molecular structure of glucose. The lines represent chemical bonds between atoms. Some of the carbon (and hydrogen) atoms are at junctions between two bonds.
The OH groups appearing in the diagram are called hydroxyl groups (hydrogen, H, is bonded to oxygen, O). Changes in the positions of the hydroxyl groups characterize different sugars. For instance, when the two hydroxyls are located at the top instead of at the bottom, the sugar represented is galactose, which has different properties and sweetness levels, even though the chemical formula, C6H12O6 ,is identical to that of glucose.
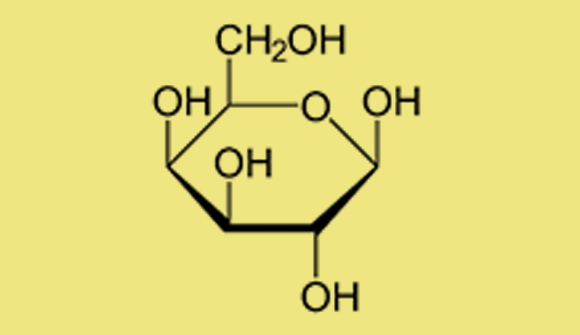
Haworth projection of the molecular structure of galactose. Tiny changes in the structure create a different compound
This phenomenon, whereby two compounds have the same chemical formula but a different physical molecular structure (and consequently different properties), is called isomerization. Each hydroxyl group can be at the top or the bottom and the rings can be pentagonal and hexagonal, so the number of isomers of glucose—and sugars in general—is very large. Indeed, the sugar family is enormous.
When two monosaccharide molecules bind to each other, they form a disaccharide. The sugar maltose, for instance, is found in barley malt (mentioned in the video). It forms when two glucose molecules bond. Sucrose, which we use as a food and drink sweetener, is the combination of glucose and fructose. Lactose, a sugar found in milk, forms when a glucose molecule links up with a galactose molecule.
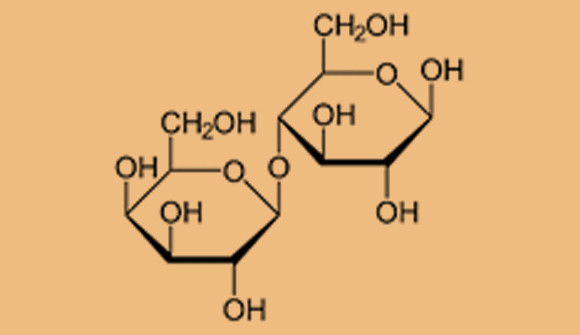
Haworth projection of the molecular structure of lactose. Formed when glucose and galactose are joined
Hence, it follows that if the number of possible monosaccharides is large, then the number of possible disaccharide combinations is even greater.
And when more than two sugar molecules combine, we have a polysaccharide. Many more than two, in fact: Polysaccharides consist of hundreds to hundreds-of-thousands of monosaccharides linked together (polysaccharides containing 3–10 sugars are also referred to as oligosaccharides, or simple sugars.) The three most common polysaccharides are starch, produced by plants to store energy and found in large quantities in wheat flour, rice, potatoes and corn; cellulose, an important structural component of plant cell walls (and main component of wood), which is in fact, the most common organic compound on the planet; and glycogen, which serves for energy storage in animals.
A section of a starch chain—hundreds of glucose molecules linked together | Source: Wikipedia Creator: Laghi.l
How Sweet
In terms of their sweetness, all the monosaccharides and disaccharides mentioned are perceived as sweet, but each to a different degree. The table below lists the relative sweetness of different compounds to sucrose, which is assigned the sweetness of 1.
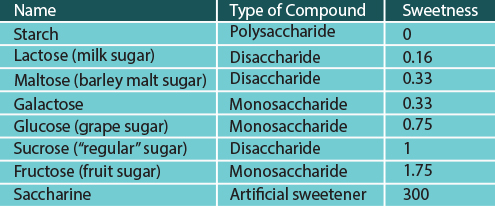
The relative sweetness of different sugar compounds
Starch is not sweet at all. By contrast, artificial sweeteners, such as saccharine (which is not a carbohydrate at all), can taste much sweeter than sugar.
If you chew on a piece of bread for a minute or two without swallowing, you will notice that the bread becomes sweeter over time. That happens because our saliva contains a compound called amylase, which breaks down the tasteless starch into its smaller constituents. Amylase belongs to a family of compounds called enzymes—chemical “construction workers” used by our body to execute and accelerate almost all the chemical reactions it needs. The amylase in saliva finishes its work when it has broken down the starch into units of two bonded glucose molecules, i.e., the disaccharide maltose. Maltose is not as sweet as table sugar; to obtain a sweetness equivalent to one teaspoon of table sugar, we would need three teaspoons of maltose.
Into the Body
As far as the body is concerned, only monosaccharides may enter through the digestive system; anything larger, even disaccharides, is blocked by the intestinal wall and does not pass into the body without breaking down. All sugar breakdown in the body is executed by enzymes, so if a specific enzyme is lacking or deficient, we will not be able to digest its target sugar. For example, humans do not have an enzyme that breaks down cellulose; thus we cannot digest straw (which consists mainly of cellulose). However, we do have the enzyme that breaks apart lactose, so we can digest the sugar in milk. However, in many people, the body’s production of this enzyme decreases with age and therefore, the consumption of lactose-containing milk can cause a disruption in the digestive system as we grow older, because of the presence of a substance we cannot digest in the milk.
Digesting Starches
Starches are difficult to digest (that is, to break down), but during pre-historic times, humans discovered fire and cooking, which help us digest starch properly. In general, long starch chains in plants are in a “crystalized” state—dense structures which the body’s enzymes have difficulty penetrating and breaking down. When cooking starches, we typically add water and apply heat (think about making dough or rice). This process causes the long, crystalized starch chains to spread out, making them easier to digest. If we have just pure starch in water, as I did in the experiment, or when preparing corn flour porridge, malabi, or salep, this chain opening is clearly visible and leads to the formation of a thick liquid.

An illustration of the thickening process that occurs when a mixture of starch and water is heated. Animation: Avi Saig
That was the first stage in my preparations for the breakdown of starch in the video clip. After grating and mashing the potatoes, I washed the potato starch to the bottom of the bowl, added boiling water, and turned it into a porridge. I then waited for the mashed potatoes to cool down, before adding the enzyme amylase; as enzymes are proteins that are sensitive to high temperatures; just like egg whites, which change their structure when exposed to heat.
As mentioned earlier, the full breakdown of starch by amylase produces maltose, which is not particularly sweet. Our relatively short process yielded just a partial breakdown, which resulted in a mixture of maltose and short starch chains. To enhance its sweetness, I conducted another breakdown process, using the enzyme amyloglucosidase (also known as gamma-amylase or glucoamylase). Each enzyme has a pH level (the level of acidity/alkalinity) at which it is active. The amyloglucosidase I used functions optimally in slightly acidic conditions, at a pH of 4.5. That why I added a few drops of lemon juice to the solution (I also measured the pH level, but that is not shown in the clip).
The breakdown—in the human body as in the bowl—takes a few hours. To maximize efficiency, factories manufacturing glucose syrup from corn starch allow the enzyme to work a few days. It should also be mentioned that as the enzymes work away, they need water to help break down the long starch molecule. In other words, rather than a shearing-off process, it is a water-assisted breakdown reaction (called hydrolysis).

The enzyme amyloglucosidase uses water to break down starch | Illustration: Naama Ziv Hayoun
I waited three hours for breakdown, then concentrated the sugar by boiling and evaporating the water I had added earlier, not before adding baking soda, an alkaline, to neutralize the acidity of the added lemon juice, as I did not want an acidic sugar syrup. Finally, I had a small dish of sweet sugar syrup!
The result illustrates the fact that carbohydrates and sugar are one and the same. Ultimately, all carbohydrates break down into a syrup consisting of simple monosaccharides. So when you eat a bowl of rice, a plate of mashed potatoes, or cornflakes with milk, what is actually entering your body is a bowl of sugar syrup, a plate of sugar, or milk with sugar.
Are Carbohydrates Healthy?
That’s a good question: On the one hand, no one would consider eating large quantities of the final product of the carbohydrate digestion process all day long. We do not consume entire bowls of sugar on a daily basis, especially as high sugar intake intensifies diseases such as obesity and diabetes.
On the other hand, polysaccharides are not monosaccharides. They do not reach the bloodstream immediately; they must first undergo a lengthy digestion process, which provides the body with “fuel” in a measured manner over several hours. Moreover, the enzymes needed to break down carbohydrates and starches are present in our saliva and intestines, which means that our bodies are built for this type of nutrition. On top of that, human saliva contains amylase at eight times the concentration found in chimpanzee saliva, our closest evolutionary relative. Genetically speaking, the number of copies of the amylase manufacturing gene in humans is triple that of chimpanzees. All of these facts indicate that historically, starch digestion has provided us human beings with an evolutionary advantage. Those with a better ability to digest starch survived over time.
And speaking of history—numerous historians have noted that the ability to store grains, with their high starch content, was conducive to the emergence of human civilization: The rice civilization in eastern Asia; the barley/wheat civilization in Europe, and the corn (maize) civilization in Central and South America. A whole year’s reserve of food gathered over a single month paves the way to free time, and also creates the need for many different types of organizers and leaders with tasks other than food production, from building grain stores to the construction of agricultural tools. So perhaps carbohydrates should be credited for their role in bringing humanity to where it is today?
